World's First Engineered T Cell Receptor Trial Opens with New Cellular Therapy for HIV
First ever clinical trial with engineered T cells designed to clear HIV infection
October 8, 2009 - (Oxford, UK and Philadelphia, PA) - Researchers at Adaptimmune Limited and the University of Pennsylvania School of Medicine,
today announced the approval of an Investigational New Drug (IND) application from the US Food and Drug Administration (FDA) and opening for enrollment of the first ever study using patients'
cells carrying an engineered T cell receptor to treat HIV (SL9 HA-GAG-TCR). The trial may have important implications in the development of new treatments for HIV potentially
slowing - or even preventing - the onset of AIDS.
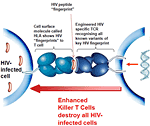 The trial makes use of the body's natural ability to recognize infected cells by enhancing the power of the T cell receptor (TCR) on killer T cells. When a virus infects cells, it hijacks the host cell machinery in order to replicate and spread infection. These infected cells then expose or
"present" small parts of the virus proteins on their surface, offering a "molecular fingerprint" called an epitope for killer T-cells from the immune system to identify. This triggers
an immune response, eliminating the virus and any cells involved in its production. However, HIV not only replicates itself quickly on infection but also has the ability to mutate rapidly, swiftly disguising its fingerprints to allow it to hide from killer T-cells. The trial makes use of the body's natural ability to recognize infected cells by enhancing the power of the T cell receptor (TCR) on killer T cells. When a virus infects cells, it hijacks the host cell machinery in order to replicate and spread infection. These infected cells then expose or
"present" small parts of the virus proteins on their surface, offering a "molecular fingerprint" called an epitope for killer T-cells from the immune system to identify. This triggers
an immune response, eliminating the virus and any cells involved in its production. However, HIV not only replicates itself quickly on infection but also has the ability to mutate rapidly, swiftly disguising its fingerprints to allow it to hide from killer T-cells.

Researchers at the Oxford University spin-out Adaptimmune have spent a decade working on ways to improve the natural ability of the TCR to recognize infected and cancerous cells; a process which has involved remaking the natural TCR protein and then modifying its ability to bind to the molecular fingerprints of the affected cells.

Last year, with colleagues at the University of Pennsylvania, they engineered and tested a killer T-cell receptor that can recognize all the different disguises HIV is known to have used to evade detection . The researchers transferred this receptor to the killer T-cells to create genetically engineered "bionic assassins" able to destroy HIV-infected cells in culture.
Now, less than a year later, they are taking their unique technology into the clinic.
"The immune system uses T cell receptors to find and trigger the elimination of infected cells", says Dr Bent Jakobsen, Chief Scientific Officer at Adaptimmune
Ltd. "HIV, however, poses an intractable challenge because it has a phenomenal ability to escape detection through mutation whilst the immune system is not able to
adapt its T cell receptors. Together with our colleagues at the University of Pennsylvania, we have previously shown that it is possible to engineer a T cell receptor
that detects the known spectrum of HIV escape mutants for this particular fingerprint and triggers a more potent immune response when transferred into a patient's
cells. Today sees that important research result move into the clinic - for the first time allowing us to test the power of super potent immune cells against HIV in reality."
The trial will be led by Carl June, MD of the Abramson Family Cancer Research Institute and the
Department of Pathology and Laboratory Medicine with Pablo Tebas, MD , Director of the adult
AIDS Clinical Trials Unit (ACTU), Department of Infectious Diseases Division at the University of Pennsylvania.
Professor June said: "We are treating patients for the first time with an enhanced version of a natural T cell receptor designed to recognize and clear HIV. This is the
first time an engineered T cell receptor will be given to patients with HIV infection. We will be treating patients currently well-controlled on HAART therapies in order
to establish whether the engineered killer T cells containing the receptor are safe, and to identify a range of doses of the cells that can be safely administered.
It is a truly an important day for T cell immunotherapy."
"Using monoclonal antibodies revolutionized the treatment of many rheumatologic and lympho proliferative diseases", added Tebas, Principal Investigator on the new
trial. "These engineered T cells are the equivalent of monoclonal antibodies in the other big branch of the immune system: the cellular branch. We hope to target cells infected
with the HIV virus and eliminate them. This first study will evaluate safety and the right dose of these cells needed to be effective."
According to UNAIDS/WHO figures, over 2.7 million people were infected with HIV in 2007 with over 33 million people estimated to be living with HIV worldwide. No cure or
effective vaccine yet exists. Current treatment regimens are based on combinations of different classed of anti-retroviral drugs which although successful in delaying the onset of
AIDS for several years, have serious side effects and must be taken daily for life. Drug resistance is also increasingly a problem. New, effective ways to control the disease
therefore remain a priority.
If the trial confirms the safety and preliminary effectiveness of the engineered T cell treatment for HIV, Adaptimmune plans to conduct a follow-on Phase II trial to
confirm efficacy in a larger group of patients. In partnership with its colleagues at the University of Pennsylvania it is also planning a first safety study of engineered T cells
targeted to cancer to commence in 2010.
Note: The Penn investigators in this study have no financial interest or other relationship with Adaptimmune LTD, apart from their scientific collaboration
in developing the engineered killer T cell, conducting laboratory experiments and planning human clinical trials.
HIV-infected patients interested in learning more about the trial should contact Larisa Zifchak at: +1 215-349-8091 ( larisa.zifchak@uphs.upenn.edu )
The SL9 HA-GAG-TCR Trial:
The study is an open label, exploratory Phase I multiple arm study to evaluate the safety, tolerability and antiviral effects of escalating doses of a single administration of autologous T cells
modified using lentiviral vectors expressing high affinity gag-specific TCRs in patients who are positive for the tissue type HLA A02. The specific target of the TCR is the HIV-1 gag epitope
SL9 (SLYNVATL) detectable in 75% of HIV-infected patients with this tissue type.
The process to make the autologous cells at the University of Penn will involve isolating and converting a portion of the patient's T cells to a single population of killer (CD8+) T cells
containing the engineered TCRs. Two TCRs will be compared: the natural wild type TCR for the SL9 epitope (WT-GAG-TCR) and a higher affinity version (HA-GAG-TCR). The trial will
enroll a total of twenty-four patients who are currently well-controlled on anti-retroviral medications (HAART) in four equal cohorts. In the first cohort, patients
will be infused with WT-GAG-TCR T cells and then start an analytical structured treatment interruption (STI) to their HAART for a maximum of 16 weeks unless
criteria for reinstatement of HAART are invoked. After initial dosing, patients will enroll in the second cohort evaluating HA-GAG-TCR T cells. Once a
safe dose is established in these two cohorts, the third and fourth cohorts will enroll in which eligible patients will begin an STI and then
receive T cell infusions 6 weeks in to their STI of initially WT-GAG-TCR (cohort 3) and then HA-GAG-TCR (cohort 4), unless criteria for
HAART reinstatement are invoked. All patients will be followed for 9 months following study treatment. The primary objective of
the study is to evaluate the safety and feasibility of treating patients with HA-GAG-TCR modified T cells.
Preclinical data
Preclinical data on SL9 HA-GAG-TCR were published in the journal Nature Medicine (Varela-Rohena, A. et al. Control of HIV-1 immune escape by CD8 T-cells expressing enhanced T-cell receptor.
Nature Medicine, 2008 Dec;14(12):1390-5.) and presented at the European AIDS Conference in Vilnius in April 2009. Phage display was used to isolate and enhance a T-cell antigen receptor (TCR)
originating from a CTL line derived from an infected person and specific for the immunodominant HLA-A(*)02-restricted, HIVgag-specific peptide SLYNTVATL (SL9). High-affinity (KD < 400 pM) TCRs were
produced that bound with a half-life in excess of 2.5 h, retained specificity, targeted HIV-infected cells and recognized all common escape variants of this epitope. CD8 T cells transduced with this
supraphysiologic TCR produced a greater range of soluble factors and more interleukin-2 than those transduced with natural SL9-specific TCR, and they effectively controlled wild-type and mutant strains
of HIV at effector-to-target ratios that could be achieved by T-cell therapy.
About HIV/AIDS
Since the discovery of the human immunodeficiency virus (HIV) in 1984 and its role in the cause acquired immunodeficiency syndrome (AIDS) the HIV pandemic has become one of the most serious
challenges to human health in the 21st Century. UNAIDS estimates indicate that over 33 million people are now living with HIV rising by approximately 1 million per year.
Whilst combinations of highly active anti-retroviral therapy (HAART) have been relatively successful in crippling the virus and delaying by years the onset of AIDS,
crucially such therapy does not represent a cure and the combined problems of drug resistance mutations, toxicity and patient adherence raise questions about
the long-term efficacy of treatment as well as the cost and availability of such drugs in poorer parts of the world where the pandemic is most acute.
More recently, hopes that vaccines could be used to control the disease by provoking an immune response to the virus have also begun to fade as
it has become apparent that HIV's phenomenal capacity for variation enables it to out-run, and eventually over-run, the human immune system.
New approaches are needed that reach beyond these existing efforts, barrier methods and behavioral changes which can truly prevent or cure HIV infection.
###
PENN Medicine is a $3.6 billion enterprise dedicated to the related missions of medical education, biomedical research, and excellence in patient care.
PENN Medicine consists of the University of Pennsylvania School of Medicine (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System.
Penn's School of Medicine is currently ranked #3 in the nation in U.S.News & World Report's survey of top research-oriented medical schools; and, according to the National Institutes of Health,
received over $366 million in NIH grants (excluding contracts) in the 2008 fiscal year. Supporting 1,700 fulltime faculty and 700 students, the School of Medicine is recognized worldwide
for its superior education and training of the next generation of physician-scientists and leaders of academic medicine.
The University of Pennsylvania Health System (UPHS) includes its flagship hospital, the Hospital of the University of Pennsylvania, rated one of the nation's top ten "Honor Roll" hospitals by
U.S.News & World Report; Pennsylvania Hospital, the nation's first hospital; and Penn Presbyterian Medical Center, named one of the nation's "100 Top Hospitals" for cardiovascular care by Thomson Reuters. In addition UPHS includes a primary-care provider network; a faculty practice plan; home care, hospice, and nursing home; three multispecialty satellite facilities; as well as the Penn Medicine at Rittenhouse campus, which offers comprehensive inpatient rehabilitation facilities and outpatient services in multiple specialties.
Related Links
Source: Penn Medicine
http://www.uphs.upenn.edu/news/News_Releases/2009/10/engineered-t-cell-hiv-trial/
|