News Release
14-JUL-2023
Researchers solve structure of immune-evading HIV protein complex
The research may help develop antiviral therapy against HIV/AIDS in the future
OKINAWA INSTITUTE OF SCIENCE AND TECHNOLOGY (OIST) GRADUATE UNIVERSITY
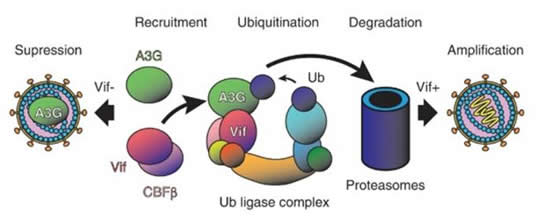
CAPTION: When encapsidated in budding HIV-1 virions, APOBEC3G (A3G) suppresses viral amplification by modifying the viral genome after reverse transcription in infected cells (left). But HIV-1 Vif catalyzes degradation of A3G, leading to infectious virions (right).
CREDIT: OIST
The HIV-1 virus can neutralize cellular defenses with its “viral infectivity factor (Vif)”. OIST researchers, Prof. Matthias Wolf and Dr. Takahide Kouno together with an international team of colleagues have now determined the atomic structure of the “APOBEC3G-Vif complex” using cryo-electron microscopy.
“APOBEC3G (A3G) is a key component of the human innate immune system to defend against invading viruses, getting a ride inside budding virions like in a Trojan horse so that it can modify and disable viral DNA after reverse transcription in infected cells,” Prof. Wolf, the senior author of the study and who leads the OIST Molecular Cryo-Electron Microscopy Unit explains. “But HIV-1 has evolved a counteraction mechanism in the form of its Vif protein, which inhibits this process by binding to and degrading A3G, leading to successful amplification of infectious viral particles.”
A unique aspect of their study is the first demonstration that this complex formation is mediated by specific RNA sequences, even though single-stranded DNA is the substrate of degradation by A3G. Their protein construct was optimized for higher solubility and could replicate the degradation pathway by A3G ubiquitination (a process that involves the transferof ubiquitin to a target proteina and is important in many physiological functions) in vitro.
The work identified specific interactions between protein and the RNA ligand, which may be targeted with drugs for future antiviral therapies against HIV/AIDS that inhibit the A3G-Vif interaction.
The study was published in Nature Communication on July 7th: https://www.nature.com/articles/s41467-023-39796-5
JOURNAL
Nature Communications
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
Lab-produced tissue samples
CONTACTS
Tomomi Okubo
Manager, Media Relations Section
Communication and PR Division
Okinawa Institute of Science and Technology (OIST)
www.oist.jp
Source: https://www.eurekalert.org/news-releases/995606
"Reproduced with permission - Okinawa Institute of Science and Technology (OIST)"
Okinawa Institute of Science and Technology (OIST)
For more HIV and AIDS News visit...
Positively Positive - Living with HIV/AIDS:
HIV/AIDS News |